Search
Highly Enantio‐ and Diastereoselective Synthesis of β‐Methyl‐γ‐monofluoromethyl‐Substituted Alcohols
- TCHLab
- Jun 7, 2011
- 1 min read
Wenguo Yang, Xinle Wei, Yuanhang Pan, Richmond Lee, Bo Zhu, Dr. Hongjun Liu, Dr. Lin Yan, Prof. Dr. Kuo‐Wei Huang, Prof. Dr. Zhiyong Jiang, Prof. Dr. Choon‐Hong Tan
Chemistry - An European Journal, 2011, 17, 8066–8070 (VIP – Very Important Paper)
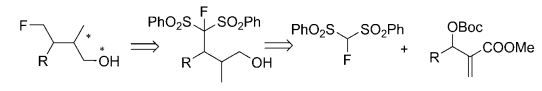
Abstract: Enanatiopure β‐methyl‐γ‐monofluoromethyl alcohols were prepared from the allylic alkylation between fluorobis(phenylsulfonyl)methane with Morita–Baylis–Hillman carbonates. The reaction was catalyzed by using the Cinchona alkaloid derivative, (DHQD)2AQN. The origin of the stereoselectivity was verified by DFT methods. Calculated geometries and relative energies of various transition states strongly support the observed stereoselectivity.
Paper download:




Comments