Search
Amino-Indanol Catalyzed Enantioselective Reactions of 3-Hydroxy-2-Pyridones
- TCHLab
- Jan 24, 2009
- 1 min read
Julian Ying-Teck Soh and Choon-Hong Tan
J. Am. Chem. Soc. 2009 13 6904-6905 (Highlighted by Synfacts, 2009, 7, 0792-0792; contributors: Benjamin List, Saihu Liao)
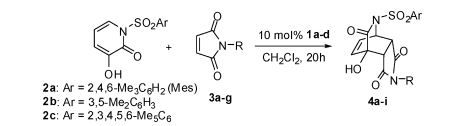
Abstract: A new bifunctional catalyst, chiral amino-indanol 1a, containing both Brønsted base and hydrogen bonding donor moieties, has been identified. It is easily prepared in a single step from commercially available amino-indanol. It was found to be an excellent catalyst for Diels−Alder reactions of both 3-hydroxy-2-pyridone and 3-hydroxy-2-pyrone. Various dienophiles including N-substituted maleimides were investigated. The Diels−Alder adducts were obtained in excellent yields and high enantioselectivities.
Paper download:




Comments